Phosphate Production
Reshoring Phosphate Production for the American Manufacturing Renaissance
by Laura Lammers, PhD
Phosphorus: The Most Critical Element?
Phosphorus is an essential element required for life. While the nutrient nitrogen can be created from the atmosphere by microbes in soil, phosphorus comes from minerals bound up in rocks. Once released, phosphorus is available to plants for only a short time before being immobilized in non-bioavailable forms.
While new research is laying the groundwork for more-efficient phosphorus utilization in soils (cf. Basinski et al., 2024), the nutrient phosphorus today must be mined, refined, and applied as fertilizer to support agriculture. Natural cycling of phosphorus is not sufficient to sustain the global population. Fully half of the world’s agricultural output relies on synthetic phosphorus, with an even higher proportion in Western Europe, Asia, and North America (Demay et al., 2023).
Phosphorus is also a key element in the energy sector. Several of the lowest-cost battery chemistries, such as lithium iron phosphate (LFP) and sodium vanadium phosphate for sodium-ion batteries (NIBs), require phosphorus. Today, LFP batteries dominate electric vehicle and stationary battery energy storage markets, leading to a 40% compound annual growth rate for EV battery-grade phosphate demand (DOE, 2023). Phosphorus is critical to modern energy storage and delivery of low-cost, quickly deployable, and reliable grid electricity.
Despite its importance to food and energy security, the US government has removed phosphorus from its lists of critical materials. The justification for its exclusion by the US Department of Energy (DOE) was based on a narrow definition of criticality and a faith in overseas producers meeting US demand. By the current definition, only elements that are considered to a) have a high risk of supply chain disruption and b) are deemed essential in energy technologies are included in the list (DOE, 2025).
Phosphorus was evaluated in the US DOE Critical Materials Assessment in 2023 but excluded from the list because of “the ability of the US to expand its phosphorus imports” (DOE, 2023). Imports can theoretically be supplied by friendly producers based in India, Saudi Arabia, or Morocco. However, a substantial fraction of US imports of purified phosphoric acid originate from the same country that dominates the supply of other minerals deemed critical: China. This decision had national security and food security implications, because exclusion from the list limits the availability of Federal funding to develop phosphate projects. In November, the Department of the Interior added phosphates to its Critical Minerals Assessment List for 2025 based on new data, interagency recommendations, and public feedback. This emphasizes the critical need the US has for domestic sources of phosphates to meet the growing demands of many key industries.
The continued exclusion of phosphorus from the critical materials list, in favor of reliance on overseas imports, will harm American competitiveness in producing this chemical for essential agricultural and energy applications.
The Flight of Phosphate Production from America: a Waste Problem
The conventional process for phosphorus extraction from rock, the wet phosphoric acid (WPA) process, was developed more than a century ago (Gilmour, 2014) and produces 3–5 tons of an industrial chemical waste known as phosphogypsum (PG) per ton of phosphoric acid produced (King & Moates, 2013; Figure 1). The produced phosphoric acid can be used to make phosphate fertilizers, purified further to make LFP batteries, or purified even further to make acid for food and beverage products like Coke and Pepsi. The waste phosphogypsum, on the other hand, creates a massive environmental challenge.
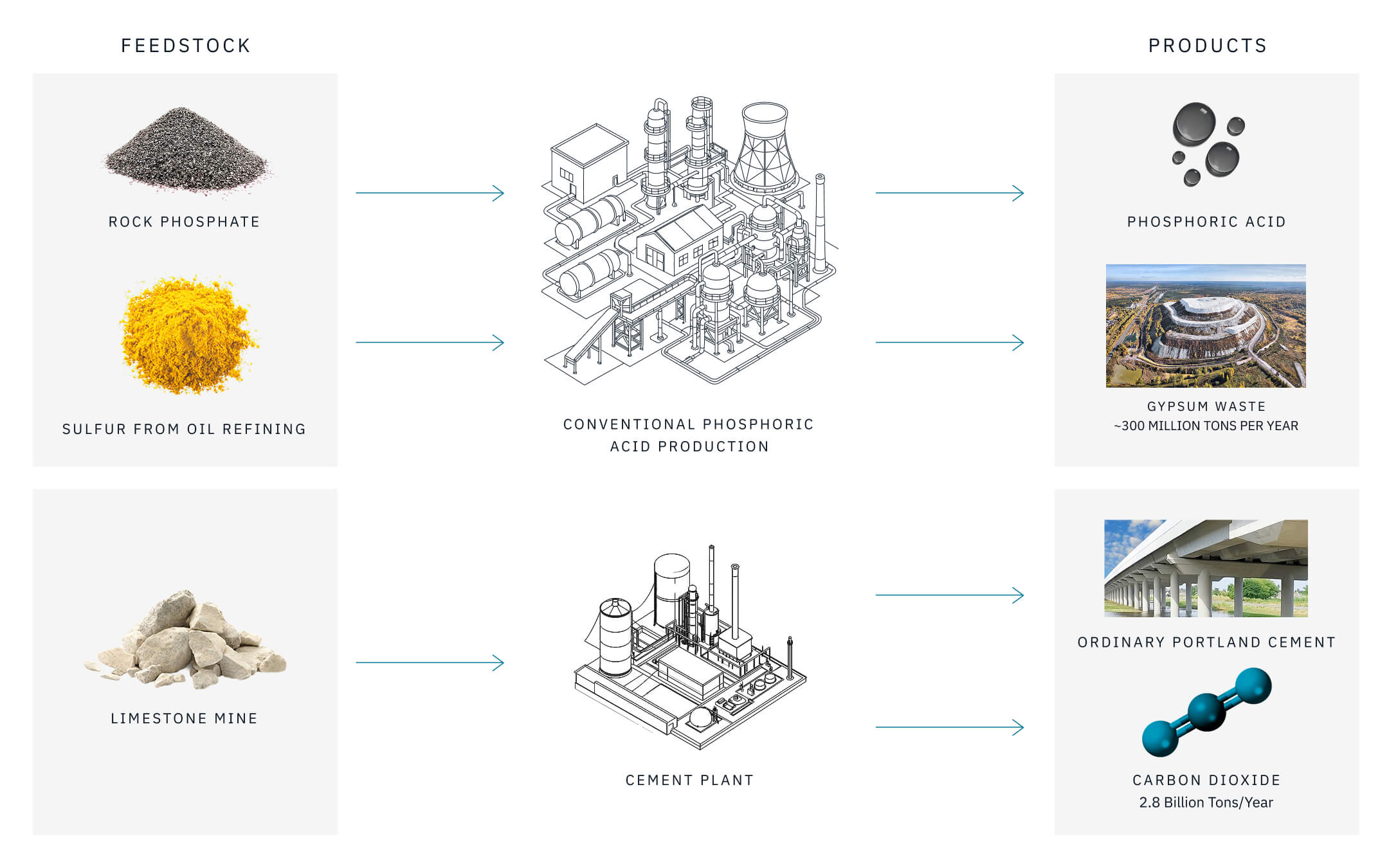
Phosphogypsum is unsuitable for conventional uses of gypsum such as drywall manufacturing because it contains residual acids, silicate minerals, and trace hazardous elements. A small amount can be used in cement or in agriculture, but those use cases are insufficient to stem the tide of new waste production, so the vast majority of PG is landfilled. Since the invention of phosphate fertilizers, billions of tons of phosphogypsum waste have accumulated, and the amount increases by around 300 million tons every year (King & Moates, 2013).
In the United States, phosphogypsum is subject to strict regulations that make new phosphate plants nearly impossible to permit. US regulations require PG to be landfilled in engineered “stacks,” which contain recirculating water that needs to be treated for decades. Rainfall and wastewater addition on the stacks means that they accumulate millions of gallons of contaminated, acidic water per year, all of which must be treated to meet strict water quality standards for discharge. The magnitude of cost is evident from public filings from phosphate producers; phosphogypsum producers are responsible for hundreds of millions of dollars in closure costs (cf. Mosaic, 2023). New stack installation at operating sites costs upwards of $100M USD.
As a result, no major new American phosphate plants have been built since 1975.

Despite their burden and cost to producers, even these regulations fall short of what in other industries would be considered appropriate treatment. In 1980, the Bevill Amendment temporarily exempted phosphogypsum from regulation under the Resource Conservation and Recovery Act (RCRA), not because landfilling the material is not environmentally hazardous, but rather because its designation as such would prevent producers from producing phosphates at a reasonable price. The fact that phosphorus can be produced in the United States at all is the result of an unsatisfying — and unsustainable — environmental compromise.
The consequence of the phosphogypsum problem is the flight of phosphoric acid production overseas to countries where environmental and labor protections are often less strong.
US phosphate nutrient exports have declined from an average of ~250,000 t/yr in 2010 to ~150,000 t/yr in 2024. The US now imports as much phosphate nutrient as it exports, and our reliance on offshore supply continues to grow (Argus Direct, 2024). The gap is driven by declining competitiveness of domestic phosphate production since the 1980s, due in part to a very expensive waste management problem.
A Brief History of Phosphate Production Technology
The wet phosphoric acid (WPA) process has dominated phosphoric acid production in the world for over a century, driven by an abundant supply of waste sulfur from fossil fuel refining (Gilmour, 2014). In this process, sulfuric acid and process heat are supplied by sulfur burning. Phosphate rock is fed to a reactor that digests the rock into phosphoric acid using sulfuric acid, producing gypsum-rich residue. Phosphoric acid produced in the digester is reconcentrated and treated to remove impurities, including residual sulfate, fluorine, and certain metals, before reaction with ammonia to produce fertilizers, most commonly monoammonium and diammonium phosphate (MAP and DAP).
This process was developed in its original form (Figure 1) after World War I, over a century ago, and only relatively minor technological improvements, such as the invention of tilting pan filters, have made it to the production line.
Alternative production methods have been developed that address one or more of the WPA process problems, but none has seen broad commercial adoption. In World War II, scarcity of sulfur led to the development of the Müller-Kühne Process (Faucher et al., 2025), which thermally recycles gypsum into sulfuric acid and lime (CaO). Unlike phosphogypsum, this lime is suitable for large-scale utilization in cement as a replacement for calcium carbonate in the production of clinker, provided radiological risk standards can be met.
Although the process produces useful lime instead of phosphogypsum waste, it suffers from a high energy consumption due to the high temperature of gypsum thermal decomposition (>1,000°C). Furthermore, addition of coke as a sulfate reductant creates large amounts of carbon emissions in the process, while use of sulfur as an alternative reductant produces excess sulfuric acid (Faucher et al., 2025).
The combined challenges of waste, cost, and unacceptable carbon emissions means none of these existing technologies meet the needs of the current phosphoric industry in North America.
Major producers are unable to build new WPA plants due to the environmental hazards — and resulting liability costs — created by phosphogypsum production. As a result, American producers are forced to shut plants down or repair plants that are decades past their reasonable lifespan. Most recently, a large phosphoric acid plant was decommissioned in Geismar, Louisiana, and no plants have been built to make up the lost capacity.
Travertine’s Carbon-Negative Process for Phosphate Production Without Phosphogypsum Waste
In 2022, Travertine was founded to solve the dual problems of industrial sulfate waste and industrial decarbonization (Lammers et al., 2023). Sulfuric acid is the most-used inorganic chemical in the world, and globally, more than 50% is used to produce phosphates (King & Moates, 2013). Instead of producing sulfuric acid by sulfur burning or thermal processes, Travertine’s process electrochemically recycles sulfuric acid to deliver savings and sustainability. Travertine’s core process converts magnesium or calcium sulfate wastes, such as phosphogypsum, into sulfuric acid and magnesium or calcium carbonate using carbon dioxide captured from the air or point sources. To meet the needs of the modern phosphate industry, Travertine has developed a continuous electrochemical process for phosphoric acid production (Figure 3; patents pending).
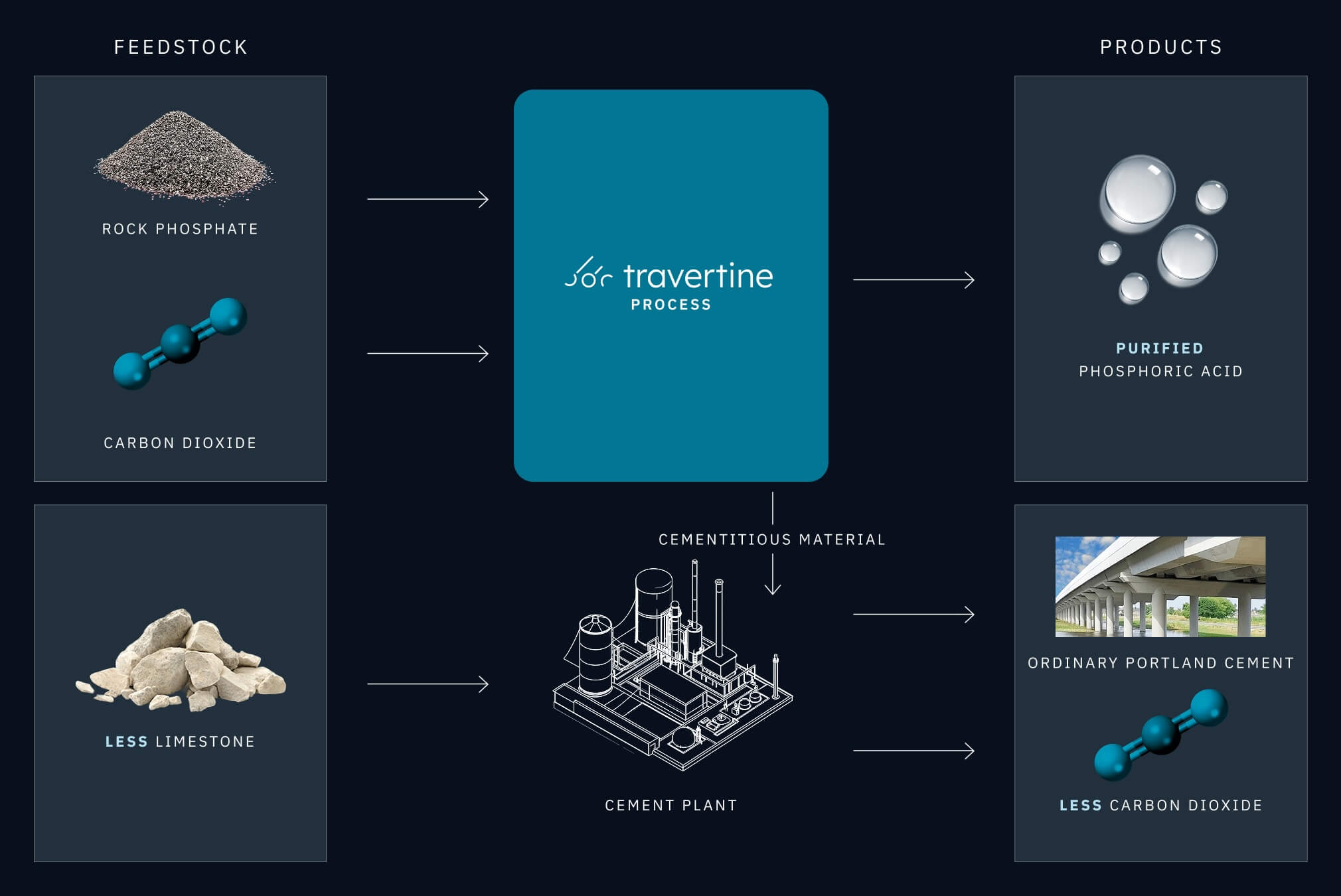
Travertine’s phosphate process produces phosphates from rock phosphate ore using recycled sulfuric acid to eliminate phosphogypsum waste, while producing carbon-neutral or carbon-negative materials for cements (Figure 3). Steps of the process include electrolysis, (optional) CO2 capture, rock digestion, mineralization, and (optional) phosphoric acid purification to food or battery-grade products.
At scale, we believe Travertine can produce phosphate products at comparable cost to the WPA process, while eliminating the longstanding environmental cost and liability and definite social cost of building and managing phosphogypsum stacks.
When powered by renewable electricity, Travertine’s process is carbon-negative, sequestering 0.75 tons of carbon dioxide (gross) per ton of phosphoric acid produced. Replacing conventional WPA with this process could result in >1 billion tons of carbon dioxide abatement or removal from the atmosphere in 10 years.
Travertine’s technological and process innovations can help solve the problems of conventional phosphoric acid production, allowing us to bring new plants back to North America.
Argus Direct. US Phosphate Fertilizer Tradeflow: Nutrient-level
Import Balance. Argus Media Group (2024).
Basinski, J.J., Bone, S.E., Klein, A.R. et al. “Unraveling iron oxides
as abiotic catalysts of organic phosphorus recycling in soil and sediment matrices.” Nat Commun 15, 5930 (2024). https://doi.org/10.1038/s41467-024-47931-z
Demay, J., Ringeval, B., Pellerin, S. et al. “Half of global agricultural soil phosphorus fertility derived from anthropogenic sources.” Nat. Geosci. 16, 69–74 (2023). https://doi.org/10.1038/s41561-022-01092-0
DOE, 2023. https://www.energy.gov/sites/default/files/2023-05/2023-critical-materials-assessment.pdf
DOE, 2025. https://www.energy.gov/cmm/what-are-critical-materials-and-critical-minerals
Faucher, S., Shaner, M., Omelchenko, S., Anikeeva, G., and McKay,
I. ACS Sustainable Chem. Eng. 2025, https://doi.org/10.1021/acssuschemeng.4c08838
Gilmour, R. Phosphoric Acid: Purification, Uses, Technology, and
Economics. Taylor & Francis Group, LLC, 2014.
King, M.; Moats, M.; Davenport, W. G. Sulfuric Acid Manufacture:
Analysis, Control and Optimization. Newnes, 2013.
Lammers, L.N., Duan, Y., Anaya, L., Koishi, A., Lopez, R., Jassby, D. and Sedlak, D.L. (2023) “Electrolytic Sulfuric Acid Production with Carbon Mineralization for Permanent Carbon Dioxide Removal.” ACS Sustainable Chem. Eng., 11, 12, 4800–4812. https://doi.org/10.1021/acssuschemeng.2c07441.
Mosaic, 2023. The Mosaic Company: Calendar Year 2023 Financial Review. Available online: https://investors.mosaicco.com/financials/annual-reports/default.aspx.
